Abstract
Background: Efforts to augment the efficacy of cell therapies include development of 4th generation chimeric antigen receptor (CAR) T-cells delivering a transgenic activator protein to the tumor target. Interleukin-18 (IL-18) is a pro-inflammatory cytokine shown to enhance CAR T-cell proliferative potency and antitumor activity in pre-clinical models (Hu et al, Cell Reports 2017).
Methods: We are conducting a first-in-human trial using huCART19-IL18, a 4th generation autologous CAR T-cell product transduced by lentiviral vector to co-express humanized anti-CD19 CAR and IL-18 in patients (pts) with relapsed/refractory B-cell non-Hodgkin lymphomas (NHL) or chronic lymphocytic leukemia (CLL) (NCT04684563). We are using a Bayesian optimal interval dose titration design exploring doses between 3 and 300 million huCART19-IL18 cells per pt. The ex vivo culture time for manufacturing is reduced to 3 days to further improve T-cell activity/persistence and to shorten the time from apheresis to treatment. For this ongoing phase 1 trial, pts must be ≥ 18 years old, have CD19+ relapsed/refractory B-cell NHL or CLL, and have had at least 2 lines of therapy including failure of prior CAR T-cell therapy (if indicated by FDA label). The primary objective is to define the recommended phase 2 dose and evaluate the safety of huCART19-IL18; secondary objectives are feasibility, efficacy, and characterization of pharmacokinetics. Following apheresis, optional bridging therapy is permitted. HuCART19-IL18 cells are administered as a single IV infusion 2-5 days after lymphodepleting chemotherapy (LD). Pts with clinical benefit are eligible to receive retreatment. Dose-limiting toxicity (DLT) observation period is 28 days after infusion. Responses are assessed using Lugano criteria for NHL and revised iwCLL criteria for CLL at months (M) 3, 6, 9, and 12.
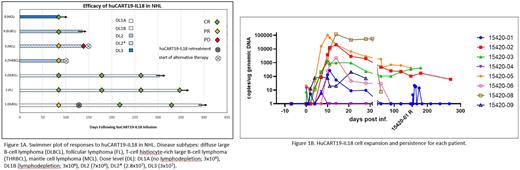
Results: As of July 14, 2022, 9 pts have enrolled, and 8 pts have been infused and are evaluable for safety (DLBCL 3, MCL 2, THRBCL 1, HGBL 1, FL 1). At enrollment, median age was 65 years (56-75), 75% were males, median ECOG PS was 1 (0-1), the median number of prior therapies was 6.5 (range 4-13), 7/8 (88%) pts relapsed after prior CAR-T (3 post axi-cel, 3 post tisa-cel, 1 post brex-cel). The best responses to prior CAR T-cell therapy were CR in 3, PD in 3, PR in 1. Seven pts (88%) received systemic bridging therapy including 5 (63%) who also had radiation. Pts receiving LD were treated with bendamustine (90 mg/m2 x 2 days). With staggered enrollment to allow for DLT evaluation, the median time from apheresis to infusion was 47 days (26-82). The first pt was infused with DL1A (3x106 cells) without LD. Subsequently, 2 were infused with DL1B (3x106 cells), 1 with DL2 (7x106), and 2 with DL3 (3x107), all after LD. Manufacturing for 2 products did not meet the target dose but exceeded minimum infusible dose and pts were treated: 1 with DL2 (7x106) and 1 with dose between DL2 and DL3 (2.8x107). Cytokine release syndrome (CRS) occurred in 4 (50%) pts: Grade (G)1 in 2, G2 in 1, G3 in 1 with median onset at 7.5 days (2-8) and median duration of 5.5 days (5-11); 2 pts required anti-cytokine therapy. Neurotoxicity occurred in 2 (25%) pts: 1 on day 20 lasting for 2 days (G1) and 1 on day 8 for 6 days (G2). Other non-hematologic G3 or higher adverse events at least possibly related to huCART19-IL18 included infections in 2 (25%), hypotension in 2 (25%), and AST elevation in 1 (12.5%) in the setting of CRS. There have been no study-related deaths. Of 7 pts who are evaluable for response (DLBCL 3, THRBCL 1, MCL 2, FL 1), the ORR at M3 is 100% (CR 57%, PR 43%). Of the 3 pts with PR at M3, 1 pt was re-treated with huCART19-IL18 at M4 (achieved CR at M3 after re-treatment and remains in CR), 1 pt was taken off study in PR to pursue alternative therapy, and 1 pt progressed at M5 with CD19-negative disease and is receiving alternative therapy. None of the 4 pts who achieved CR at M3 have progressed to date including FL pt refractory to axi-cel who is in sustained CR over 12 M after huCART19-IL18 at DL1B (Figure 1A). All pts are alive at median follow-up of 8 M (1.9-14.1). Figure 1B shows huCART19-IL18 cell expansion and persistence for each pt.
Conclusions: In this first-in-human study, huCART19-IL18 shows a manageable toxicity profile and encouraging early efficacy across all dose levels in heavily pretreated pts with CD19+ NHL including those who did not respond to prior 2nd generation CAR T-cell products. Enrollment continues at DL3.
Disclosures
Svoboda:TG: Research Funding; SEAGEN: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Merck: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Consultancy; BMS: Consultancy, Research Funding; Atara: Consultancy; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADCT: Consultancy. Gerson:Abbvie: Consultancy; Loxo Oncology: Research Funding; Genentech: Consultancy. Landsburg:Curis, Inc: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; Calithera: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Triphase: Research Funding. Chong:Beigene: Consultancy; Tessa: Consultancy; Juno/BMS: Consultancy; KITE: Consultancy; Novartis: Consultancy. Barta:Janssen: Other: Independent Data Monitoring Committee member; Kyowa Kirin: Consultancy, Honoraria; Acrotech: Honoraria; Seagen: Honoraria; Affimed: Consultancy; Daiichi Sankyo: Consultancy. Dwivedy Nasta:Roche: Research Funding; FortySeven/Gilead: Research Funding; Rafael: Research Funding; Pharmacyclics: Research Funding. Ruella:Beckman Coulter: Research Funding; BMS: Consultancy; GlaxoSmithKline: Consultancy; Bayer: Consultancy; viTToria Biotherapeutics: Other: Scientific Founder, Patents & Royalties: related to CD19 CAR T cells; NanoString: Consultancy, Research Funding; AbClon: Consultancy, Research Funding. Hexner:Blueprint Medicines Corporation: Consultancy, Research Funding; PharmaEssentia: Consultancy; Samus Therapeutics, Novartis Oncology: Research Funding; American Board of Internal Medicine: Other: Member of the hematology exam committee; Tmunity Therapeutics: Research Funding. Davis:Novartis Institute for Biomedical Research: Patents & Royalties; Cellares Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tmunity Therapeutics: Patents & Royalties, Research Funding. Porter:DeCART: Consultancy; BMS: Consultancy; Bluebird Bio: Consultancy; Kadmon: Consultancy; Angiocrine: Consultancy; Mirror Biologics: Consultancy; Genentech: Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company; Tmunity Therapeutics: Patents & Royalties: anti-CD19 CART; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Wiley: Honoraria; Elsevier: Honoraria; Adecept Bio: Consultancy; Jazz: Consultancy; Janssen: Consultancy; Gerson Lerhman Group: Consultancy; Incyte: Consultancy; Kite/Gilead: Consultancy; Novartis: Consultancy, Patents & Royalties: anti-CD19 CART, Research Funding. Schuster:Merck: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Juno Therapeutics: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Nordic Nanovector: Consultancy; Loxo Oncology: Consultancy; TG Therapeutics: Research Funding; DTRM: Research Funding; Adaptive Biotechnologies: Research Funding; Roche: Consultancy, Research Funding; AstraZeneca: Consultancy; Fate Therapeutics: Consultancy; Genmab: Consultancy; Mustang Biotech: Consultancy; BeiGene: Consultancy; Pharmacyclics: Research Funding; Regeneron: Consultancy; MorphoSys: Consultancy; Novartis: Consultancy, Honoraria, Patents & Royalties: related to CD19 CAR T cells , Research Funding; Incyte: Consultancy, Research Funding; Legend Biotech: Consultancy. June:Tmunity Therapeutics: Other: Co-scientific Founder, Patents & Royalties: Co-inventor of anti-CD19 CART, Research Funding; AC Immune: Membership on an entity's Board of Directors or advisory committees; BluesphereBio: Consultancy; Cabaletta: Consultancy; Carisma: Consultancy; Cellares: Consultancy; Capstan Therapeutics: Other: Co-scientific founder, Research Funding; Alaunos: Consultancy, Other: Founder; Poseida: Consultancy; Verismo: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal